Grignard reagents
And their reactions
Reagent formation - standard procedure
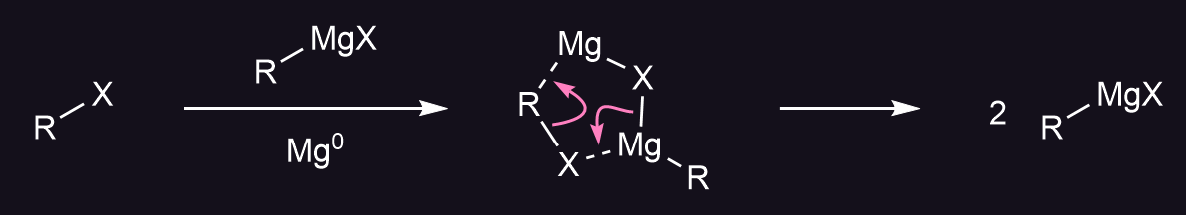
A grignard reagent1 is an alkyl/aryl-magnesium halide. Grignards are in many ways similar to organolithium reagents, although I shall only focus on the former here. It is usually made by the reaction of magnesium and an organohalide; the reaction mechanism is not totally understood and involves a complex mixture of reactive species called a Schlenk equilibrium23 that react through both nucleophilic and radical mechanisms4.
Note that the magnesium gets oxidised from the 0 to the +2 oxidation state (oxidative insertion). This exothermic reaction takes place on the surface of the metal, thus the ease of formation of grignard reagent depends on the state of the surface. There is usually a layer of oxide on the metal, causing an induction period for the reaction. To prevent a runaway reaction, the halide is added gradually.

This process can be sped up by initiating the reaction with the addition of some crystals of iodine, 1,2-diiodoethane (which forms bubbles of gaseous ethene upon reaction, a useful indicator) or using ultrasound to dislodge the oxide layer. Once the reagent starts to form, it catalyses further reactions:
The reaction is usually carried out in ethereal solvent such as ether or THF. A small amount of mercuric chloride will amalgamate the surface of the metal, enhancing its reactivity. A small amount of inorganic nitrate salt also supposedly accelerates the reaction5. Sublimating the magnesium under inert atmosphere will produce highly reactive crystals that lack an oxide layer. Another trick to speed up the reaction is “Rieke magnesium”6, a highly reactive form of the metal lacking an oxide layer that is produced on heating a magnesium salt with an alkali metal under inert atmosphere. The addition of a small amount of KI potentiates this reaction7.
The presence of even a small amount of water will both destroy the reagent (through an acid-base reaction) and significantly lengthen the induction period.
Agitation also has a large impact on induction period.
From what I can tell the purity and particle size of the magnesium does not matter greatly8, although from past experience I have had trouble starting reactions with a very small mesh size. Heating the magnesium prior to reaction will help to ensure its dryness.
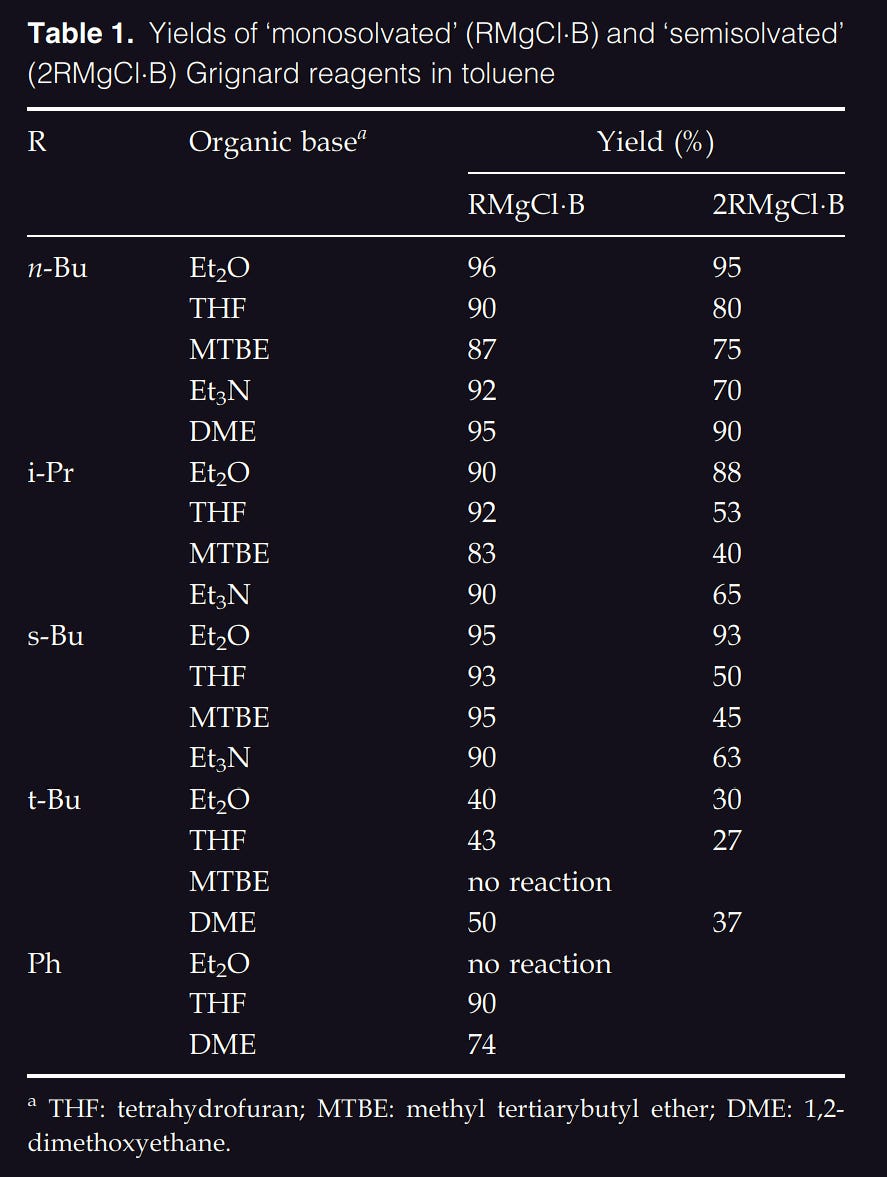
Chloroalkanes are less reactive and thus it is harder to form grignard reagents out of them. This can be overcome by using a mixture of bromo- and chloro- reactants9. Alternatively, a solvent mixture containing toluene and the use of sonication has proven to be highly effective10:
The reagents were prepared in a flask equipped with a mechanical stirrer. The reaction vessel was charged with 3-4 g of magnesium turnings (Fluka, 99.8% Mg), 30 ml toluene, and the appropriate amounts of donor solvents. The magnesium metal was activated prior to use by dry heating with few milligrams of iodine. The reaction was initiated with a little of the halide dissolved in the donor and the rest was then added dropwise. The temperature was maintained slightly below the boiling point of the halide. The reaction was complete in 1-2 h. Ultrasonic irradiation shortened the reaction time by a factor of two or more, but it did not influence the yields (the formation of Wurtz-coupling by-products).
Reagent formation - alternative methods
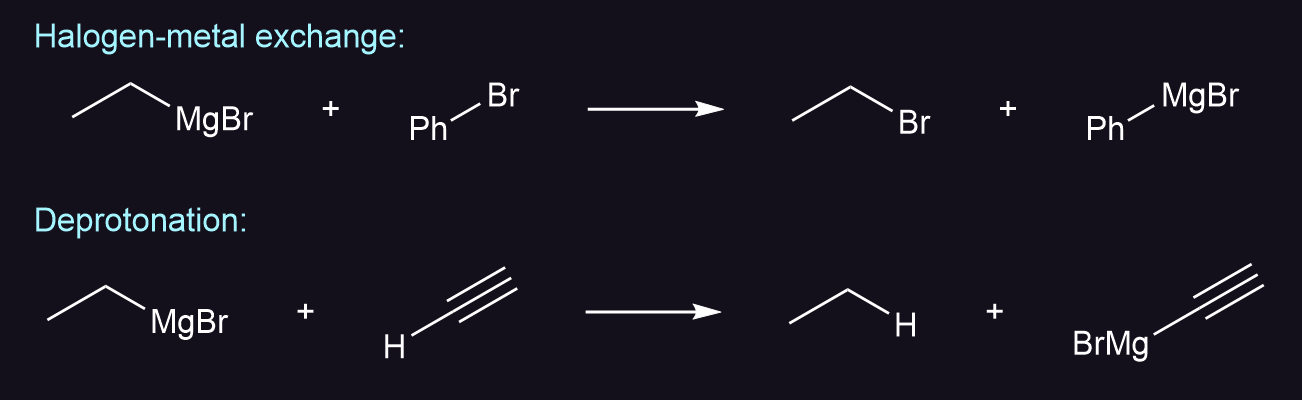
A grignard reagent can be formed by deprotonation or by halogen-metal exchange, where a more reactive grignard reacts with a less reactive species.
Note that the following reaction does not occur in any appreciable yield due to halogen-metal exchange.
This reaction can instead be carried out using a lithium organocopper (aka Gilman) reagent11 in a reaction known as the Corey–House synthesis12. Gilman reagents can be made in situ via the addition the addition of copper (I), e.g. CuCl.
A grignard reagent can be formed via transmetallation, wherein magnesium replaces a less reactive metal such as zinc.
Grignard reactions
Standard reactions
The electropositive magnesium ion “pushes” electron density onto the neighbouring carbon atom, making it a useful nucleophile that can be reacted with carbonyls to form alcohols or with CO2 (in the form of dry ice) to form carboxylic acids.
Sterically hindered substrates may react according to a single electron transfer (SET) mechanism.
An example procedure13:
Tetrahydrofuran (150 ml, commercial THF dried over 20% KOH just before use, moisture content by KF <0.10%) added to a clean dry three necked flask ( 1 Lt) carrying thermometer pocket and mechanical stirrer under nitrogen atmosphere. Magnesium turnings (14.5 g, 0.6 mol) added all at once. To this a small amount of iodine (~ 0.5-1 g) and ethyl bromide (2-3 mL, one can also use di-bromoethane) added without stirring and observed for initiation of the reaction. At this point one can see bubbles at the junction of magnesium where ethyl bromide is added. To this add about 3-5 mL of a mixture of Chlorobenzene ( 50 g, 0.45 mol) and bromobenzene (15 g, 0.10mol) in toluene (200 mL) added and observe the initiation ( sometimes slight warming of the flask is required). Once the initiation starts continue the addition of mixture of chlorobenzene and bromobenzene over period of 1 hr keeping the exothermic reaction. Do not try to cool the reaction. Keep the exothermic reaction going by the way of addition of chlorobenzene and bromobenzene. After complete addition, reflux for 2 h under nitrogen atmosphere. Cool to about 5oC and add a mixture of Benzaldehyde (53 g, 0.5 mol) in dry toluene ( 200 mL) over a period of 45 min keeping the temperature below 8 oC. After complete addition, stir for 30 min below 8oC and remove cooling. Stir for 4 h, during which the temperature comes to room temperature. Quench the reaction mixture with aqueous ammonium chloride ( ~50 g in 150 mL water)and warm to about 50-60 oC for 30 min. Add water (200 mL) and separate water layer. Water wash the organic layer again and dry over sodium sulphate (~ 75 g). Distil the organic layer under vacuum to get benzhydrol as the only product (70 g, 95% yield ) . This is normally pure by NMR
Grignard reagents can also react with esters and epoxides (where they will generally react the less sterically hindered side). If they react with carboxylic acids, they undergo an acid-base reaction, forming an unreactive carboxylate salt.
Generally speaking, grignard reagents add directly to α,β-unsaturated carbonyls, whereas Gilman reagents undergo conjugate addition.
The Barbier reaction14, which was discovered before the Grignard reaction, can be thought of as a "one-pot grignard procedure". However, unlike Grignard reagents, the organometallic species generated in a Barbier reaction are unstable and thus cannot be stored or sold commercially. These species are stable to water, and the reaction may even use water as a solvent. Victor Grignard, who eventually won the Nobel prize for his work, was actually the student of professor Philippe Barbier.
Side reactions
With particularly bulky ketones, a deprotonation may occur.
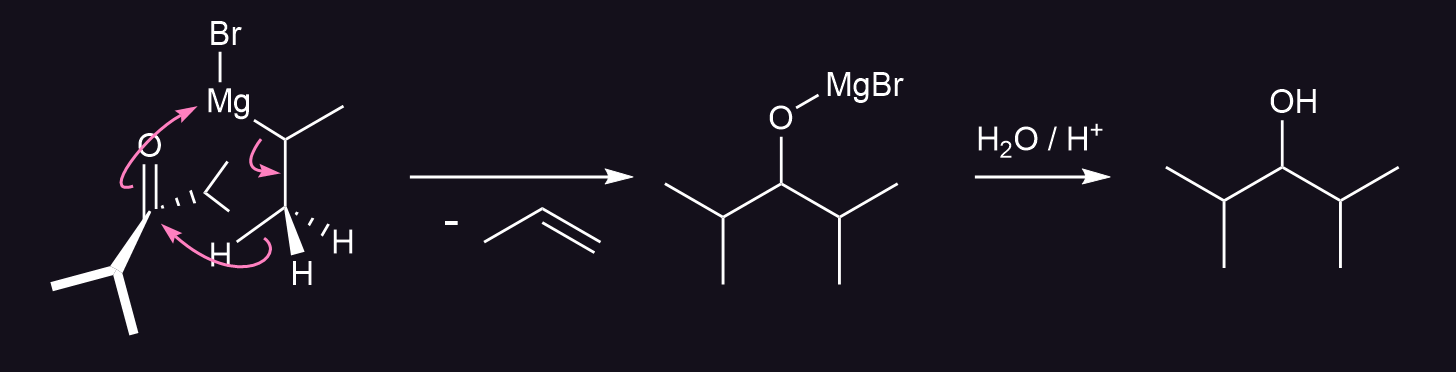
A reduction can also take place, in which a hydride is delivered from the β-carbon of the Grignard reagent to the carbonyl carbon via a cyclic six-membered transition state.
Interestingly, the addition of cerium chloride seems to increase the yield of many grignard reactions and decreases the incidence of side-reactions15.
Grignard reactions in wet solvent
A number of claims have been made of grignard or grignard-like reagents being formed or used in wet ether or even aqueous media1617181920. I haven't really looked into these but the prospect certainly seems interesting.
Reactions forming ketones or aldehydes
Organolithium reagents in excess are able to react with carboxylic acids to form ketones; normally, grignard reagents are not reactive enough to achieve this, although the reaction proceeds in good yield with the addition of i-Pr2NMgCl·LiCl21.
Alternatively, reacting exactly 1 equivalent of grignard reagent with a highly reactive carbonyl species such as an acyl chloride may produce the ketone in good yield. This reaction can produce near-100% yield if the grignard is replaced with a Gilman reagent.
To avoid the over-addition of grignard reagent in the above synthesis (causing alcohol byproduct to be formed), the Weinreb–Nahm ketone synthesis22 may be employed. Weinreb amides (made from acyl halides) form stable intermediates upon reaction with grignard reagents that are not susceptible to further addition. Weinreb amides can also be used to form aldehydes (although acyl halides may be reduced to aldehydes in one step using Rosenmund reduction23).
Primary and secondary amides get deprotonated like carboxylic acids. However, unlike RO⁻ in esters, R₂N⁻ is not a good enough leaving group in tertiary amides, so a stable intermediate is formed that produces a ketone on workup.
The reaction of grignard reagents with triethyl orthoformate produces an acetal, which can be hydrolysed to an aldehyde (known as the Bodroux–Chichibabin aldehyde synthesis).
Nitriles react with grignard reagents to form stable intermediates. Upon addition of water, the imine is formed and then subsequently hydrolysed to give a ketone.
Other reactions
Although the degradation of the grignard reagent by water is usually best avoided, it can be a convenient way of deuterating organic compounds.
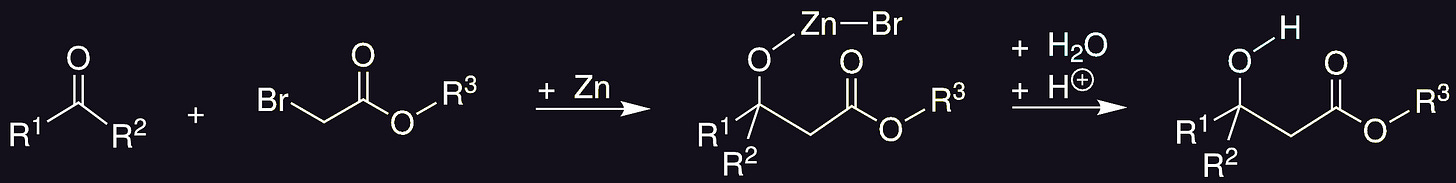
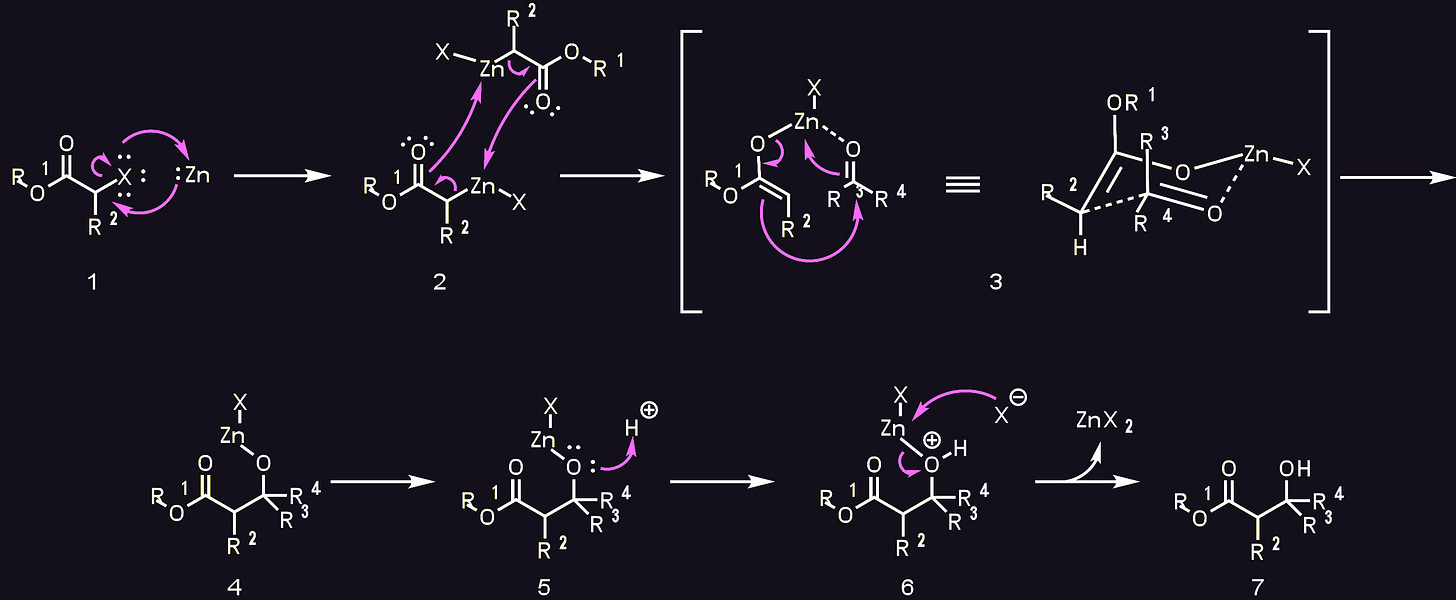
The Reformatsky reaction24 is usually carried out with zinc but has been done using many other metals including magnesium25. It forms a RMgBr species in situ, and is thus related to the grignard reaction.
At first glance the reaction may look similar to a Barbier reaction, but the mechanism is more complicated than that.
β-halo-ether grignard reagents may undergo elimination to form an alkene - this is called the Boord synthesis26.
A similar reaction can be used to produce the useful, highly reactive benzyne species2728.
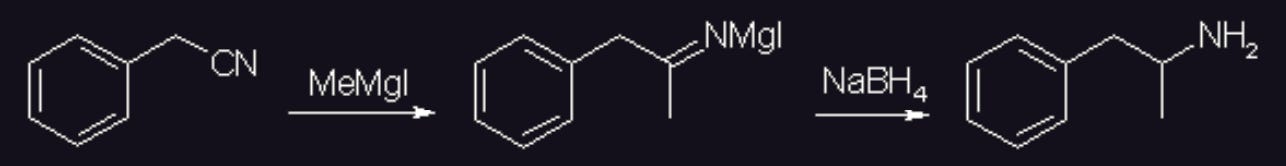
Although (for some reason that I haven’t figure out yet) this reaction is not well known, it is actually possible to reduce the intermediate formed by the addition of a grignard to a nitrile by direct addition of sodium borohydride. Similar procedures in literature all demonstrate high yield2930.
An example procedure is shown3132.
A solution of MeMgI was prepared in the usual manner from methyl iodide (15g, 0.105 mol) and clean Mg turnings (30g) in 200ml dry THF, and the solution was added slowly with good stirring to a cooled solution of (11.7g, 0.1 mol) benzyl cyanide in 150ml dry THF in a dried 1000ml round-bottomed flask. The reaction mixture was stirred at room temp for two hours and then cooled in an ice-bath. The solution was diluted with 150ml of dry methanol and NaBH4 (9.5g, 0.25 mol) was added in portions during 30 minutes, and the reaction mixture was stirred for another hour. The reaction mixture was concentrated under vacuum and the residue dissolved in 200ml water and acidified with concentrated HCl. The solution was washed with 2x50ml DCM, made basic through the addition of 25% NaOH, and extracted with 3x75ml DCM. The pooled organic extracts was dried over MgSO4, filtered and the solvent evaporated under vacuum. The oily residue was then vacuum distilled to give racemic amphetamine as a clear oil (bp 82-85°C at 13 mmHg).
Yield 10.8g (80% of theory).
What I wonder, although haven’t found any references to, is whether the intermediate may be reacted with additional alkyl halide to form substituted amines.
hm… interesting indeed
https://en.wikipedia.org/wiki/Grignard_reagent
https://en.wikipedia.org/wiki/Schlenk_equilibrium
Peltzer, R. M.; Eisenstein, O.; Nova, A.; Cascella, M. How Solvent Dynamics Controls the Schlenk Equilibrium of Grignard Reagents: A Computational Study of CH3MgCl in Tetrahydrofuran. J. Phys. Chem. B 2017, 121 (16), 4226–4237. https://doi.org/10.1021/acs.jpcb.7b02716.
Peltzer, R. M.; Gauss, J.; Eisenstein, O.; Cascella, M. The Grignard Reaction – Unraveling a Chemical Puzzle. J. Am. Chem. Soc. 2020, 142 (6), 2984–2994. https://doi.org/10.1021/jacs.9b11829.
Ramsden, H. E. Preparation of Phenyl Magnesium Chloride. US2816937A, December 17, 1957. https://patents.google.com/patent/US2816937A/en (accessed 2023-02-02).
https://en.wikipedia.org/wiki/Rieke_metal
Rieke, R. D.; Bales, S. E. Activated metals. IV. Preparation and reactions of highly reactive magnesium metal. ACS Publications. https://doi.org/10.1021/ja00813a021.
http://www.sciencemadness.org/talk/viewthread.php?tid=1900
Ramakrishna, R. Grignard addition to aldehyde via chlorobenzene metalation. https://doi.org/10.1039/SP559.
Sassian, M.; Panov, D.; Tuulmets, A. Grignard Reagents in Toluene Solutions. Appl. Organomet. Chem. 2002, 16 (9), 525–529. https://doi.org/10.1002/aoc.333.
https://en.wikipedia.org/wiki/Gilman_reagent
https://en.wikipedia.org/wiki/Corey%E2%80%93House_synthesis
https://hyperlab.info/inv/index.php?s=298e03a4ea2ab76163d76b7a85021a01&act=ST&f=17&t=28132
https://www.science.org/content/blog-post/ah-just-pour-into-salt-water
Reactions of carbonyl compounds with Grignard reagents in the presence of cerium chloride | Journal of the American Chemical Society. https://pubs.acs.org/doi/pdf/10.1021/ja00194a037 (accessed 2023-02-08).
Smith, D. H. Grignard Reactions in “Wet” Ether. J. Chem. Educ. 1999, 76 (10), 1427. https://doi.org/10.1021/ed076p1427.
Petrier, C.; Luche, J. L. Allylzinc Reagents Additions in Aqueous Media. J. Org. Chem. 1985, 50 (6), 910–912. https://doi.org/10.1021/jo00206a047.
Li, C.-J. Aqueous Barbier-Grignard Type Reaction: Scope, Mechanism, and Synthetic Applications. Tetrahedron 1996, 52 (16), 5643–5668. https://doi.org/10.1016/0040-4020(95)01056-4.
Ramakrishna, R. Grignard addition to aldehyde via chlorobenzene metalation. https://doi.org/10.1039/SP559.
https://en.wikipedia.org/wiki/Barbier_reaction
Colas, K.; dos Santos, A. C. V. D.; Mendoza, A. I-Pr2NMgCl·LiCl Enables the Synthesis of Ketones by Direct Addition of Grignard Reagents to Carboxylate Anions. Org. Lett. 2019, 21 (19), 7908–7913. https://doi.org/10.1021/acs.orglett.9b02899.
https://en.wikipedia.org/wiki/Weinreb_ketone_synthesis
https://en.wikipedia.org/wiki/Rosenmund_reduction
https://en.wikipedia.org/wiki/Reformatsky_reaction
The Reformatsky Reaction. I. Condensation of Ketones and t-Butyl Bromoacetate by Magnesium | The Journal of Organic Chemistry. https://pubs.acs.org/doi/abs/10.1021/jo01341a524 (accessed 2023-02-08).
https://en.wikipedia.org/wiki/Boord_olefin_synthesis
Heaney, H.; Mann, F. G.; Millar, I. T. 781. The Reaction of o-Bromoiodobenzene with Magnesium and Lithium. J. Chem. Soc. Resumed 1957, No. 0, 3930–3938. https://doi.org/10.1039/JR9570003930.
Wentrup, C. The Benzyne Story. Aust. J. Chem. 2010, 63 (7), 979–986. https://doi.org/10.1071/CH10179.
Lu, Z.-H.; Bhongle, N.; Su, X.; Ribe, S.; Senanayake, C. H. Novel Diacid Accelerated Borane Reducing Agent for Imines. Tetrahedron Lett. 2002, 43 (47), 8617–8620. https://doi.org/10.1016/S0040-4039(02)01905-6.
Reddy, G. O.; Sarma, M. R.; Chandrasekhar, B.; Babu, J. M.; Prasad, A. S. R.; Raju, C. M. H. A Study and Identification of Potential By-Products of Sibutramine. Org. Process Res. Dev. 1999, 3 (6), 488–492. https://doi.org/10.1021/op980093t.
https://erowid.org/archive/rhodium/chemistry/amphetamine.html
https://chemistry.mdma.ch/hiveboard/rhodium/amphetamine.html